Mutant Cells That Can’t Copy DNA Somehow Keep Dividing When They Shouldn’t – With Disastrous Consequences
Phenomenon yields “micronuclei” that are commonly found in cancer cells
Contact: Robert Perkins at (213) 740-9226 or perkinsr@usc.edu
Researchers at USC have developed a yeast model to study a gene mutation that disrupts the duplication of DNA, causing massive damage to a cell’s chromosomes, while somehow allowing the cell to continue dividing.
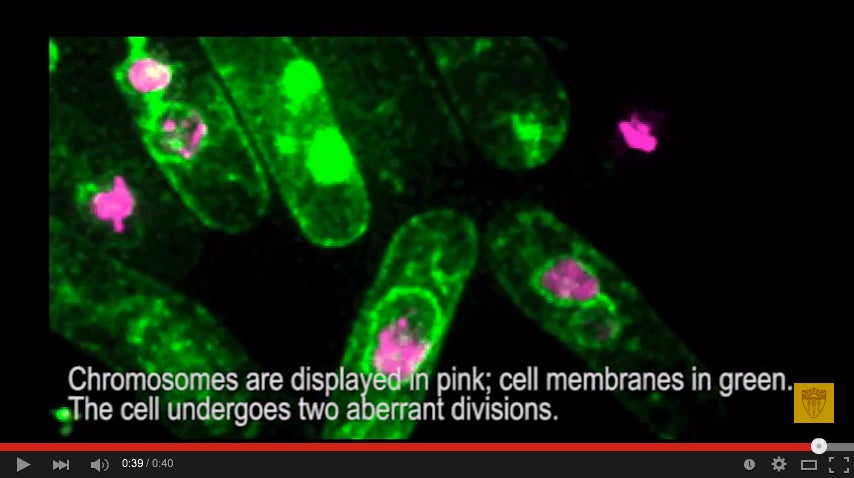
The result is a mess: Zombie cells that by all rights shouldn’t be able to survive, let alone divide, with their chromosomes shattered and strung out between tiny micronuclei. Sometimes they’re connected to each other by ultrafine DNA bridges. (Imagine tearing apart a hot pizza — these DNA bridges are like strings of cheese still draping between the separated pieces.)
Frequently, the micronuclei — which are thought to retain the most damaged portions of the DNA — rejoin the parent nuclei and incorporate mutations into the survivors.
The mutation has been associated with cancer in mice, and micronuclei are often found in human cancer cells. With their new yeast model, researchers hope to learn more about each.
“Using a simple yeast system, we have developed a powerful genetic model to investigate a recently identified characteristic of human cancer cells,” said Susan Forsburg, senior author of an article about the research that will appear online ahead of print in the journal Molecular Biology of the Cell on August 5. “This will enable us to rapidly identify genes responsible for this abnormal division.”
Since the genes that regulate division in human and yeast cells are the same, this simple organism provides a tool for human cell discovery, Forsburg said.
Video: abnormal cell divisions and resulting micronulcei (https://youtu.be/e-op9p90Fy0)
DNA is vulnerable to damage when it’s unzipped into two single strands for replication by a cell’s MCM helicase (a cellular component essential to DNA replication). Typically, the single stranded DNA triggers repair of damage by special enzymes, or — in extreme cases — drives the damaged cell to suicide. Either way, mitosis (cell division) is halted while the issue is dealt with.
But in cancer cells, despite the damaged DNA, the cells continue to divide — creating tumors full of genetic mutations. In this study, a mutation in the yeast’s MCM helicase triggered responses similar to those in mammals where mutations in this gene are associated with cancer and the formation of micronuclei.
To study the phenomenon, Forsburg and lead author Sarah Sabatinos collected videos of the damaged cells dividing so that they could maintain continuous monitoring of individual cells, and record cell division from beginning to end. They watched what happened in the mutant in real time. Then, they used a super-resolution microscope at USC that generates 3-D images of objects at the nanometer scale, to examine the damage structures in crisp detail.
“The devil’s in the division. In real time, we’re able to see that these mutant cells ignore the damage caused during DNA replication, which results in the creation of unusual structures like micronuclei,” said Sabatinos, who conducted the research as a post-doc at USC, and is now an assistant professor at Ryerson University in Toronto.
Their work will inform future studies into how a cancer cell evades biological checkpoints that should halt its division and spread.
Forsburg and Sabatinos collaborated with USC’s Nimna Ranatunga, Ji-Ping Yuan and Mark Green. Their research was supported by the National Institutes for Health, R01 GM081418 and GM111040.
The article can be found online here: http://www.molbiolcell.org/content/early/2015/08/02/mbc.E15-05-0318.abstract?sid=090e7768-cf70-4532-9924-3b2b34a453fc
Image: Sequential images of abnormal divisions in a mutant cell leading to abnormal nuclei and chromosome rearrangement. Chromosomes are displayed in pink, cell membranes in green. The cell undergoes two aberrant divisions. (Video and image courtesy of Susan Forsburg)
###